Electron Configuration Chart Aluminum
The electron configuration for aluminum is 1s2 2p2 3s6 3p1. The basis of this prediction is a rule known as the aufbau principle , which assumes that electrons are added to an atom, one at a time, starting with the lowest energy orbital, until all of the electrons have been placed in an appropriate orbital.

Pin by Dk on Chemistry Periodic table, Electron
What electron configuration matches an oxygen atom?

Electron configuration chart aluminum. There are 118 elements in the periodic table. An electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that electrons may occupy. The atomic number of aluminum is 13.
1s 2 2s 2 p 6 3s 2 p 1; A neutral atom of aluminum has 13 protons and 13 electrons. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2.
1s 2 2s 2 2p 4. It is called the box and arrow (or circle and x) orbital configuration. Value is a guess based on periodic table trend.
This means part of the electron configuration has been replaced with the element symbol of the noble gas symbol. There is yet another way to writing electron configurations. Click on 'element atomic number', 'element symbol', 'element name' and 'element electron configuration' headers to sort.
For example, krypton ends the 4th row so you would begin with 5. 1s 2 2s 2 2p 6 3s2, 3p 6 4s 2 3d 10 4p 5. 2 8 3 aluminum discovery.
The electron configuration of an atom describes the orbitals occupied by electrons on the atom. What is the electron configuration and orbital diagram of: 1s 2 2s 2 2p 6 3s 2.
Value is a guess based on periodic table trend. For example, the electron configuration of sodium is 1s 2 2s 2 2p 6 3s 1. An electron configuration can quickly and simply tell a reader how many electron orbitals an atom has as well as the number of electrons populating each of its orbitals.
Each element has a unique atomic structure that is influenced by its electronic configuration, which is the distribution of electrons across different orbitals of an atom. The electron configuration for calcium is: It was used in tanning, dyeing, and as an aid to stop minor bleeding and even as an ingredient in baking powder.
N atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. Electron configuration chart for all elements in the periodic table. Continue your electron configuration using the row after the noble gas.
24, and according to the aufbau principle, the electron configuration should be [ar]3d4s2. A shorthand way to write the electron configuration, called noble gas notation, is [ne]3^23p^1. Always begin with the s subshell.
Electron configuration [ne] 3s 2 3p 1 cas number: The ground state electron configuration for aluminum is 1s^22s^22p^63s^23p^1. First, write out the electron configuration for each parent atom.
1s 2 2s 2 2p 6. An orbital is defined as the most probable location for finding an electron. 29 and should be [ar]3d 9 2s 2, but it has been to be determined to be [ar]3d 10 4s 1.
This list of electron configurations of elements contains all the elements in increasing order of atomic number. So although a neutral atom of aluminum has 13 electrons, the ion of aluminum, al 3+, has lost three electrons and only has 10. Continue writing your electron configuration following the chart until you reach the correct number of electrons.
Aluminum will lose three electrons when it forms an ion. What atom matches this electron configuration? For example, the electron configuration of the neon atom is 1s2 2s2 2p6, using the notation explained below.
Value is a guess based on periodic table trend. Its electron configuration is \(1s^2 2s^2 2p^6 3s^1\). Thus, you should write the electron configuration for 10 electrons.
[ne]3s 2 3p 1 long form: To save room, the configurations are in noble gas shorthand. As we progress from lithium (atomic number=3) to neon (atomic number=10) across the second row or period of the table, all these atoms start.
Sublevels can be broken down into regions called orbitals. Value is a guess based on periodic table trend. The cornish chemist who discovered the metal, called it 'aluminum', after one of its source compounds, alum.
As an approximate rule, electron configurations are given by the aufbau principle and the madelung rule. An atom's electron configuration is a numeric representation of its electron orbitals. Notes on the electron configuration of particular elements:
Value is a guess based on periodic table trend. 1s 2 2s 2 2p 6 3s 2 3p 1 shell structure: This electron configuration chart table gives the electron configuration of all the elements of periodic table.
In this lecture we continue the discussion of quantum numbers and their use in electron configurations as well as the relationship of electron configuration to the periodic properties of the elements. Electron configuration calculator added nov 11, 2014 by brennenlb in chemistry find the electron configuration of any element on the periodic table of elements with this simple, yet very useful widget. Electron configurations of elements beyond hassium (element 108), including those of the undiscovered elements beyond oganesson (element 118), are predicted.
Each orbital holds 2 electrons. But for the ion which is al 3+ it has a positive charge,therefore electrons need to be taken away not added, also, the octet rule must. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10.
This diagram shows the electron configuration of aluminum. 1s 2 2s 2 2p 6 given : Actual experimental data shows the value to be [ar]3d 5 s 1.
The content that follows is the substance of general chemistry lecture 26. Shortly after, however, the international union of pure and applied chemistry (or iupac) stepped in, standardizing the suffix to the more conventional 'ium'. Iron is a unique element , which is around and inside us.

Oxidation States of Transition Metals Chemistry
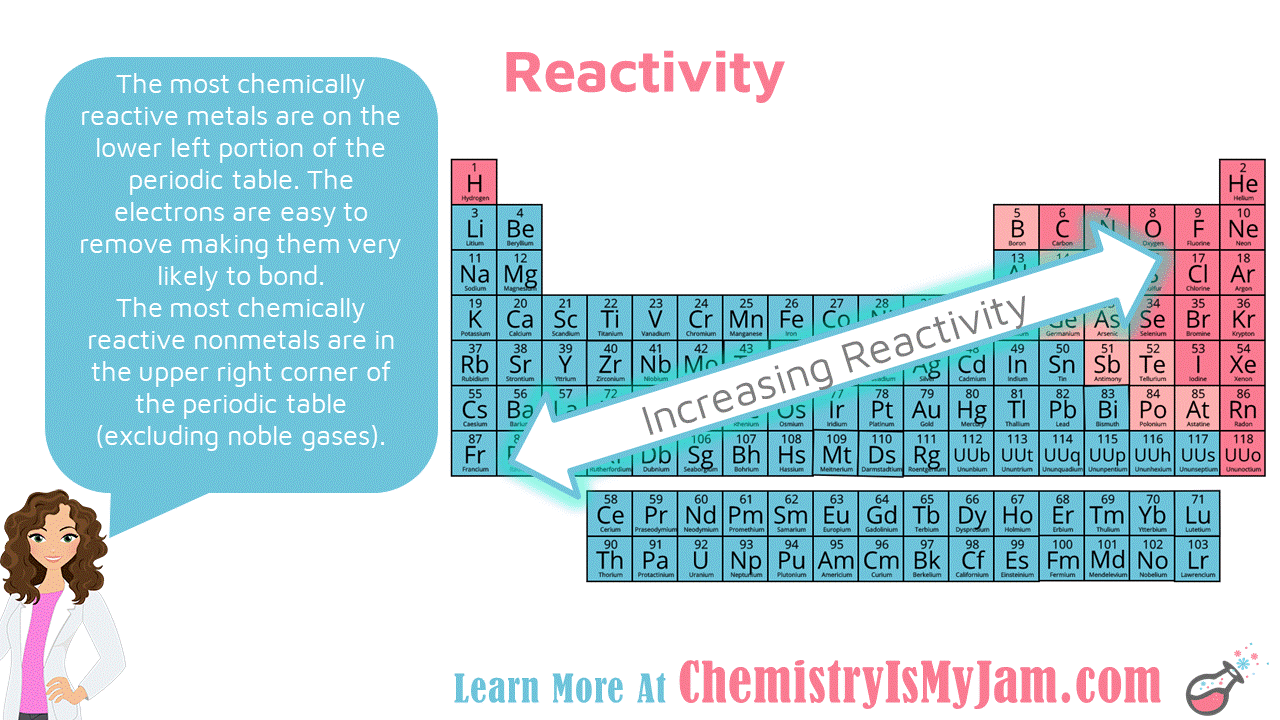
Periodic Trends Reactivity Periodic table, Electron

Periodic Geography Worksheet Geography worksheets

Best Of Periodic Table Groups Metals Metalloids and

The Alkaline Earth Metals Alkaline earth metals, Alkali

Unique Periodic Table Metals In Groups 1316 tablepriodic

Electron Configuration Chemistry lessons, Science

Iodine Art Print by Carlos Clarivan Electron

New Periodic Table Key Box (With images) Periodic table

Worksheet placed onto a powerpoint slide, revises

Tetryonics 53.84 Polonium is chemically similar to

Periodic table silver atom Necklace Electron Configuration

The Atom Bohr model, Energy level, Chemistry classroom

Periodic Table FlashChards Element Properties

FileElectron shell 039 Yttrium.svg Wikimedia Commons
Post a Comment for "Electron Configuration Chart Aluminum"